The GM1 & GM2 Tanganil Health Initiative was created in collaboration with the National Tay-Sachs and Allied Diseases Association (NTSAD) and the Cure Tay-Sachs Foundation (CTSF) to document patient experiences with off label use of Tanganil. Tanganil is a medication available without a prescription in Europe and indicated for the treatment of positional vertigo (dizziness). This Health Initiative was designed to better understand the benefits and risks experienced by patients taking Tanganil to treat symptoms associated with GM1 and GM2 disorders.
Prepared by Sarah Inoue, PhD
With help from NTSAD and CTSF, patients and caregivers who were using or planning to use Tanganil were invited to participate in this TREND Health Initiative hosted on the TREND CommunityTM platform. The participants joined a private, online community created exclusively for this Health Initiative.
DATA COLLECTION
Upon joining the TREND CommunityTM Platform, all participants consented to share their experiences and data with TREND for analysis.
Participants were asked to answer regular survey questions and keep an online journal to track their experiences with the Tanganil. All collected data were de-identified. Participants were reminded to consult with their physicians regarding any medical questions.
Approximately 20 people signed up on the platform. All were encouraged to ask questions or discuss use of Tanganil in a way that was meaningful to them. We also posed group discussion questions to encourage community building and interest.
Participants were asked to answer a series of six short surveys about Tanganil that were pre-posted into their individual journals to encourage free flow answers. These surveys focused on the following areas: Demographics, Getting Tanganil, Dose, Routine, Experience, and Comments. Eight people completed the surveys and two more sent information informally to our Team.
DATA ANALYSIS
The following is a summary of the Health Initiative results.
GROUP DEMOGRAPHICS
- 21 participants
- 6 diagnosed/14 caregivers/1 grandmother
- 11 US/3 Brazil/2 Not reported/1 India/1 Germany/1 Argentina/1 Peru
- 3 GM1/18 GM2
TAKING TANGANIL
- 10 documented
- 7 female/3 male
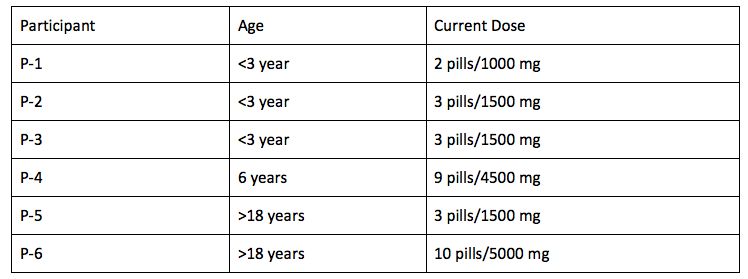
- 6 <3 years /1 six years/1 eight years/2 over 18
DOSE
Most participants are obtaining Tanganil from France and are following dosing guidelines proposed by Dr. Strupp. The cost varies based on dose size between $4 – $48 per month.
EXPERIENCES
The following anecdotes capture the range of experiences reported:
- She walks better and keeps her balance
- Able to move his neck and head
- Focus his eyes on us for a few seconds
- Smile at small stimuli
- Re-started to laugh (for 2 weeks)
- Decrease of seizures
- More alertness
- Tremors in hands improved and handwriting is legible
- Don’t drop things as much
- Strong and nasty temper
- I think my speech is slightly improved
- I am not sure it is conclusive. I do not see any special effects so far.
- It didn’t do anything for him besides give him reflux.
- It seemed like it was having GI effects and he was losing weight.
- I do not see beneficial effects.
- I don’t know if it has anything to do with Tanganil, but I have noticed an increased tremor in my hand in the past year.
UNMET NEEDS
Participants also asked questions and thoughts that should prompt further research around Tanganil, including:
- I would love to know the relationship between Tanganil and seizure reduction.
- I would like to know what is a safe level. Can I improve more by taking larger doses?
- How do I obtain Tanganil?
TREND will continue to support the community in its exploration of Tanganil as a treatment for the symptoms associated with GM1 and GM2 disorders.

